The Infusion Providers Alliance (IPA) is a leading voice for in-office and freestanding ambulatory infusion care, representing over 1,000 community-based, non-hospital providers across the United States. Our members are committed to preserving the integrity of the provider-patient relationship in a manner that delivers exceptional care to patients and value to the health care system. Our facilities are major access points of care for patients with complex and chronic health conditions, including a host of auto-immune conditions such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis as well as many other rare diseases. The convenience and exceptional patient experience in our facilities keeps these patients adherent to their medications and reduces flare ups and emergency hospital admissions.
The IPA wants to be part of the solution in reducing Medicare beneficiaries’ pharmaceutical costs, and to that end supports the following policies:
- Encouraging the migration of Part B drug administration to the most efficient setting of care;
- Tiered add-on payments, to encourage the selection of less expensive drugs when appropriate;
- A cap on beneficiary out-of-pocket expenses for Part B drugs and the ability for pharmaceutical manufacturers to provide cost-sharing assistance for these drugs; and
- Increase biosimilar adoption to fuel competition.
Encourage Migration of Part B Drugs to More Efficient Setting
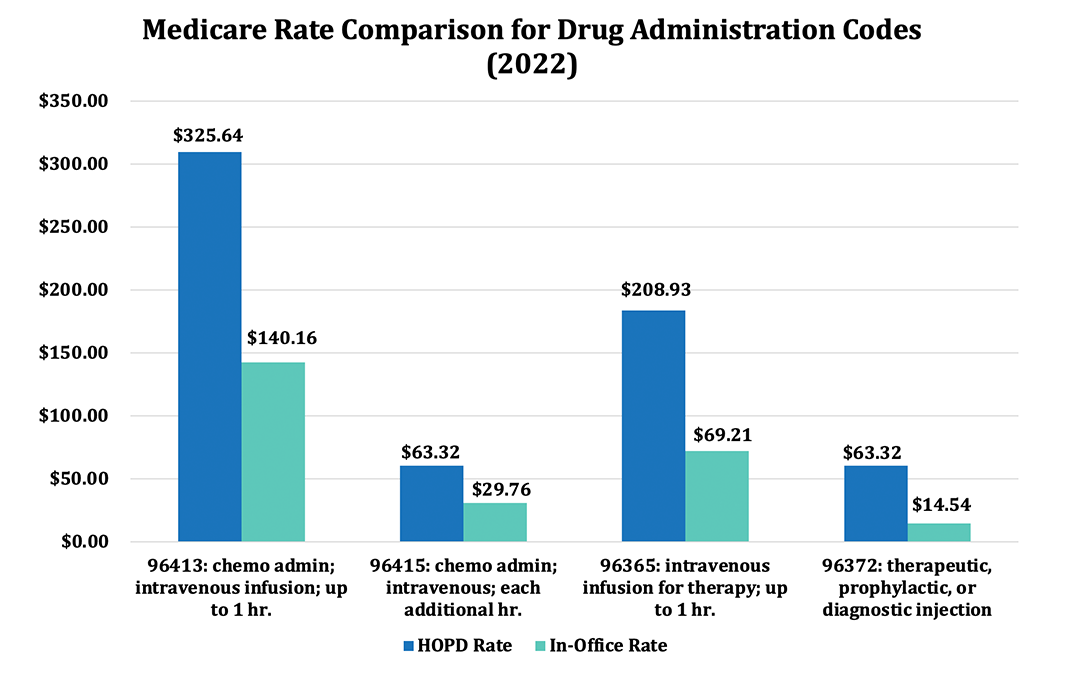
Medicare pays physician offices and freestanding infusion centers approximately half as much as hospitals for administering Part B drugs. For example, IPA infusion centers and practices regularly bill CPT code 96413 for the first hour of infusions of complex drugs such as Remicade, Ocrevus, and Entyvio for patients with a variety of diseases including Crohn’s disease, Multiple Sclerosis, and ulcerative colitis; for this particular CPT code, we are paid approximately 45 percent of the hospital rate: $309.56 vs $142.55. The contrast is even greater for CPT code 96372 for therapeutic, prophylactic or diagnostic injections such as for administering Boniva, Xolair, and Prolia for osteoporosis and arthritis: $60.46 in the hospital versus just $14.44 in the physician’s office, or 25 percent of the hospital cost.
Yet hospitals administer 44% of Part B drugs as of 2017 and their share is growing over time. From 2007 to 2017, Part B drug spending in hospital outpatient departments (HOPDs) nearly doubled, while physician offices experienced a corresponding decline.1
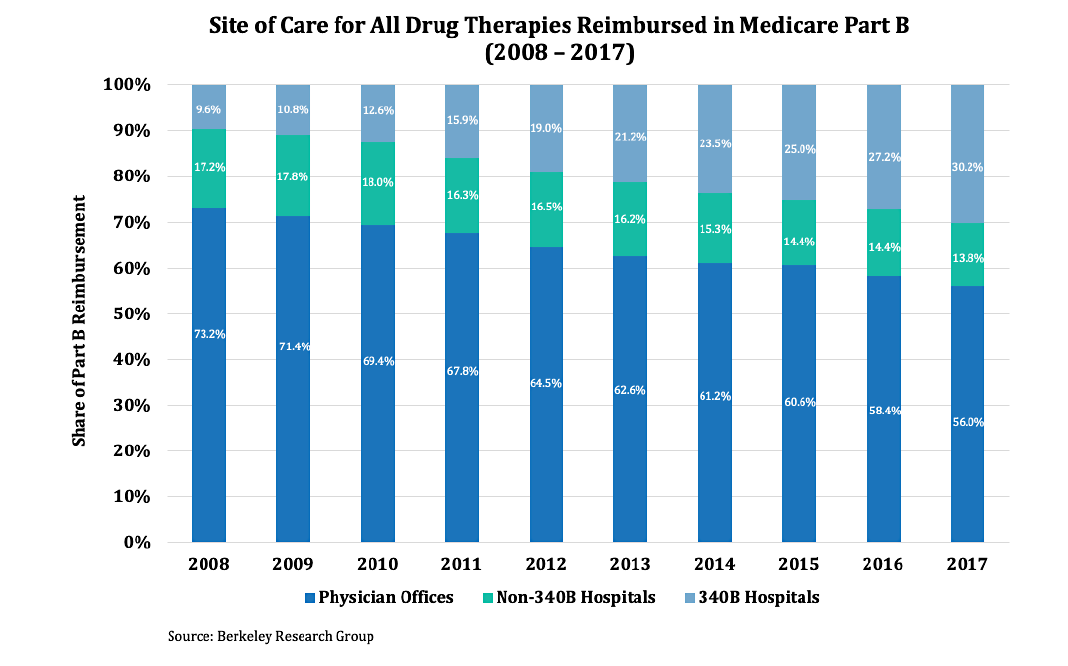
Analysis by the Office of Health Policy under the Assistant Secretary for Planning and Evaluation at the U.S. Department of Health & Human Services argues that HOPDs’ share of Part B drug spending was due to the rapid increase in payment per service relative to physicians’ offices rather than an increase of services.2The American Academy of Family Physicians commented, “Current policies that provide differential payments based solely on the site where services are provided are not warranted. Furthermore, these payment differentials create an imbalance in the marketplace that drives consolidation, reduces consumer choice and leads to higher prices for payments.”3
While Congress addressed the windfall hospitals garner for newly acquired practices under the Bipartisan Budget Act of 2015, significant underlying payment disparities persist between the two settings of care. There are several options to encourage more migration to the more efficient setting:
- Improved Transparency: Require the Medicare cost and beneficiary copayment of physician office to be publicly available via a searchable internet website.
- Improve Site Neutrality: Congress could adopt a policy that moves the two sites of service closer together without achieving 100 percent site neutrality. For example, payments to hospitals could be decreased by half of the difference between the two settings of care.
Tiered Add-On Payments
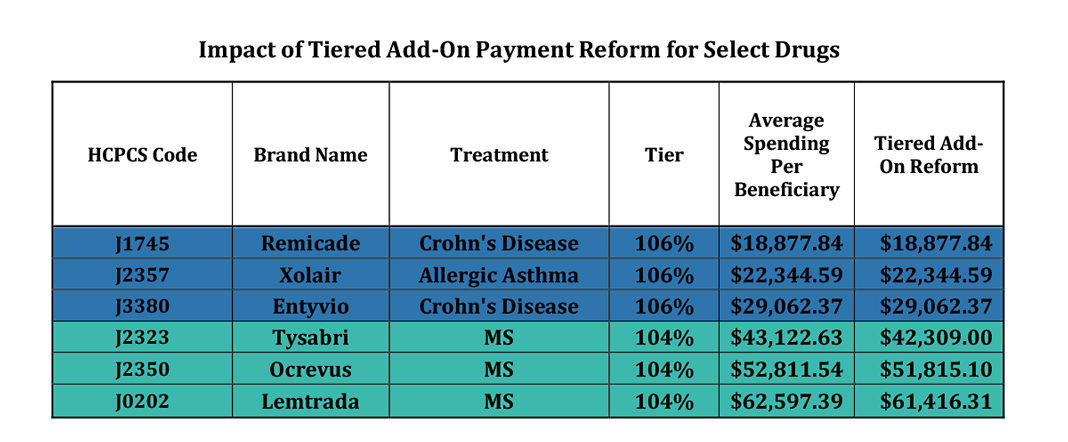
The current six percent add-on payment for Part B drugs can be reformed to better align economic incentives and provide savings to beneficiaries and the Medicare program. The current 6% add-on payment applicable to all Part B drugs could be changed to a tiered add-on payment reform, based on the cost of the Part B drug for a specified period divided by the number of Medicare beneficiaries utilizing that drug during that period. As such, the 6% add-on payment would be replaced by a new tiered approach, as follows:
» At least equal to the 85th percentile is 104%
» At least equal to the 70th percentile is 106%
» At least equal to the 50th percentile is 108%
» Less than the 50th percentile is 110%
Cap Out-of-Pocket Exposure for Part B Medicare Beneficiaries
Although the Part D drug benefit includes a catastrophic benefit, beneficiaries have unlimited out-of-pocket exposure in Part B with the 20% coinsurance. While many beneficiaries have wrap around coverage from their former employer plans or Medigap, others are left with unlimited and substantial out-of-pocket exposure. These Part B drugs, which can be very expensive and often have few therapeutic alternatives, are critical for patients as they manage their complex diseases. Moreover, unlike many lifestyle medications, the treatment regimen for these Part B drugs is tightly defined and lack of adherence can result in hospitalization. As such, IPA supports two options to assist beneficiaries; these options can work independently or in tandem:
- Create a safe harbor from the anti-kickback statute to permit manufacturers of Part B drugs to provide cost-sharing assistance to Medicare patients; and
- Provide a statutory cap for Medicare Part B drugs equal to the hospital inpatient deductible ($1,484)
Increase Biosimilar Approval and Adoption
A biosimilar pathway was created 11 years ago in the Affordable Care Act but to date only 29 have been approved and 20 are on the market. IPA supports more market-based competition for Medicare Part B drugs, including approval and market adoption of more biosimilar products. To that end, it is our belief that the tiered pricing structured referenced above would support greater adoption of biosimilar prescribing and encourage more competitive pricing by brand name manufacturers.
______________________
- Berkeley Research Group, “Site-of-Care Shift for Physician-Administered Drug Therapies: Update”
- Office of Health Policy, Assistant Secretary for Planning and Evaluation, U.S. Department of Health & Human Services,
“Medicare Part B: Trends in Spending and Utilization, 2006-2017.” - American Academy of Family Physicians Letter to President Donald Trump on “Protecting and Improving Medicare for Our Nation’s Seniors”
